Lead Carbonate Balanced Equation . the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous solutions of. balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! the balanced chemical equation has 16 carbon atoms, 36 hydrogen atoms, and 50 oxygen atoms on each side. pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes into one mole of lead. the simplest and most generally useful method for balancing chemical equations is “inspection,” better known as trial and error.
from exohqbjbg.blob.core.windows.net
balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous solutions of. the balanced chemical equation has 16 carbon atoms, 36 hydrogen atoms, and 50 oxygen atoms on each side. the simplest and most generally useful method for balancing chemical equations is “inspection,” better known as trial and error. pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes into one mole of lead.
Lead Hydroxide State Of Matter at Lester Brand blog
Lead Carbonate Balanced Equation how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous solutions of. how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous solutions of. the balanced chemical equation has 16 carbon atoms, 36 hydrogen atoms, and 50 oxygen atoms on each side. balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! the simplest and most generally useful method for balancing chemical equations is “inspection,” better known as trial and error. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes into one mole of lead.
From www.numerade.com
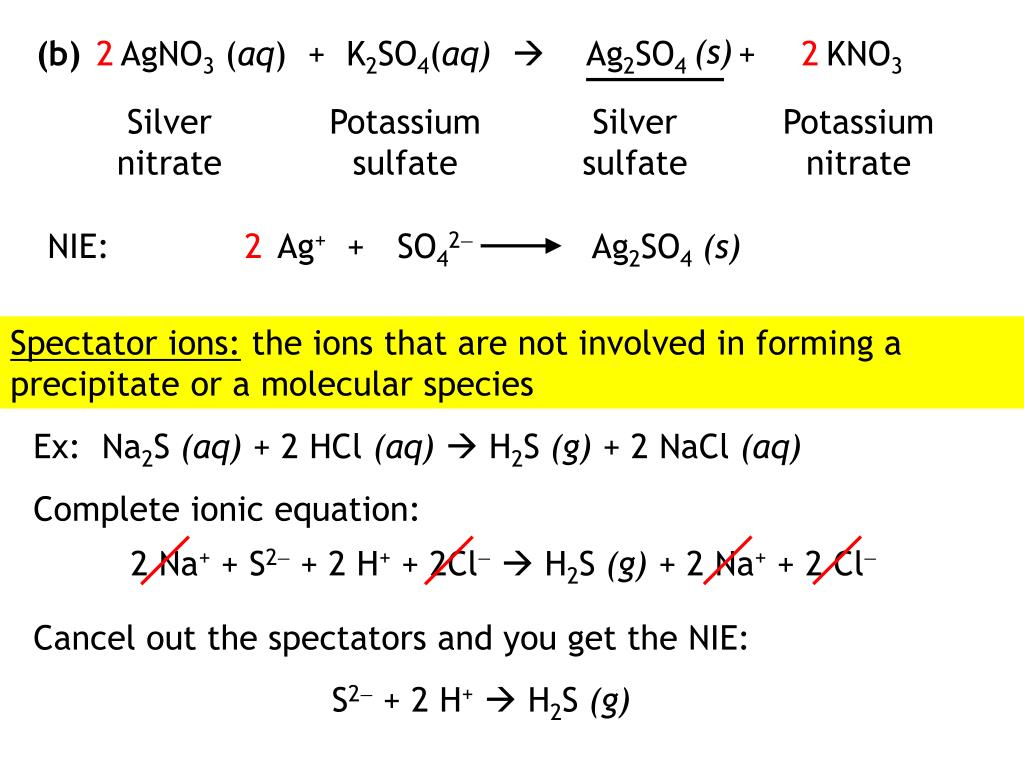
SOLVED Part Write a balanced equation for the of lead (Il) carbonate to yield Lead Carbonate Balanced Equation balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes. Lead Carbonate Balanced Equation.
From www.doubtnut.com
Give balanced equations for the following reactions Lead[II] oxide Lead Carbonate Balanced Equation the simplest and most generally useful method for balancing chemical equations is “inspection,” better known as trial and error. the balanced chemical equation has 16 carbon atoms, 36 hydrogen atoms, and 50 oxygen atoms on each side. balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! pb (co3) =. Lead Carbonate Balanced Equation.
From exocoyzqy.blob.core.windows.net
Lead In Water Chemical Equation at Gordon Maxwell blog Lead Carbonate Balanced Equation how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous solutions of. balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes into. Lead Carbonate Balanced Equation.
From exohsvhug.blob.core.windows.net
Lead Oxide And Sodium Hydroxide Equation at Sebastian Malone blog Lead Carbonate Balanced Equation balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes into one mole of lead. how do you write the balanced chemical, complete ionic and net ionic equation for the reaction. Lead Carbonate Balanced Equation.
From www.toppr.com
9 C IL DIL V A C Uw neutral gases combine to give a basic gas Certain thermal Lead Carbonate Balanced Equation the simplest and most generally useful method for balancing chemical equations is “inspection,” better known as trial and error. how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous solutions of. the balanced chemical equation has 16 carbon atoms, 36 hydrogen atoms, and 50 oxygen atoms on each side.. Lead Carbonate Balanced Equation.
From www.numerade.com
SOLVEDWrite the balanced chemical equation for each reaction. a. Solid lead(II) sulfide reacts Lead Carbonate Balanced Equation the simplest and most generally useful method for balancing chemical equations is “inspection,” better known as trial and error. balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes into one. Lead Carbonate Balanced Equation.
From cekpqokm.blob.core.windows.net
Lead Carbonate Equation For Thermal at Kathleen Rosales blog Lead Carbonate Balanced Equation the balanced chemical equation has 16 carbon atoms, 36 hydrogen atoms, and 50 oxygen atoms on each side. how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous solutions of. pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)]. Lead Carbonate Balanced Equation.
From dxolebnih.blob.core.windows.net
Lead Carbonate Complexes at Justin Diaz blog Lead Carbonate Balanced Equation how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous solutions of. the simplest and most generally useful method for balancing chemical equations is “inspection,” better known as trial and error. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element. Lead Carbonate Balanced Equation.
From www.toppr.com
Write the balanced chemical equation the following reactions & identify the of reaction i Lead Carbonate Balanced Equation balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous solutions of.. Lead Carbonate Balanced Equation.
From exouyldhc.blob.core.windows.net
Lead Carbonate + Sulphuric Acid at Harold Petersen blog Lead Carbonate Balanced Equation pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes into one mole of lead. how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous solutions of. the simplest and most generally useful method for balancing chemical equations is. Lead Carbonate Balanced Equation.
From brainly.in
Give balanced chemical equation to prepare the following salt Lead sulphate from lead carbonate Lead Carbonate Balanced Equation how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous solutions of. the balanced chemical equation has 16 carbon atoms, 36 hydrogen atoms, and 50 oxygen atoms on each side. balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! the chemical. Lead Carbonate Balanced Equation.
From www.numerade.com
SOLVED Write balanced complete chemical equation the complete ionic equation net ionic Lead Carbonate Balanced Equation how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous solutions of. balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes into. Lead Carbonate Balanced Equation.
From www.coursehero.com
[Solved] What is the balanced equation for carbonate ion (CO 3 ) acting as a... Course Hero Lead Carbonate Balanced Equation balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! the simplest and most generally useful method for balancing chemical equations is “inspection,” better known as trial and error. the balanced chemical equation has 16 carbon atoms, 36 hydrogen atoms, and 50 oxygen atoms on each side. how do you. Lead Carbonate Balanced Equation.
From exohqbjbg.blob.core.windows.net
Lead Hydroxide State Of Matter at Lester Brand blog Lead Carbonate Balanced Equation pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes into one mole of lead. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. how do you write the balanced chemical, complete ionic and net. Lead Carbonate Balanced Equation.
From www.youtube.com
How to Balance Pb(NO3)2 + NaBr = PbBr2 + NaNO3 Lead (II) nitrate + Sodium bromide YouTube Lead Carbonate Balanced Equation balance the reaction of pb(no3)2 + k2co3 = pbco3 + kno3 using this chemical equation balancer! pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes into one mole of lead. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for. Lead Carbonate Balanced Equation.
From brainly.in
c. Lead dioxide [lead (IV) oxide]= Lead monoxide + Oxygen balance Brainly.in Lead Carbonate Balanced Equation the balanced chemical equation has 16 carbon atoms, 36 hydrogen atoms, and 50 oxygen atoms on each side. pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes into one mole of lead. how do you write the balanced chemical, complete ionic and net ionic equation for. Lead Carbonate Balanced Equation.
From brainly.com
Which chemical equation describes the balanced reaction between lead and oxygen to form lead Lead Carbonate Balanced Equation the balanced chemical equation has 16 carbon atoms, 36 hydrogen atoms, and 50 oxygen atoms on each side. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. how do you write the balanced chemical, complete ionic and net ionic equation for the reaction between aqueous. Lead Carbonate Balanced Equation.
From cekpqokm.blob.core.windows.net
Lead Carbonate Equation For Thermal at Kathleen Rosales blog Lead Carbonate Balanced Equation the balanced chemical equation has 16 carbon atoms, 36 hydrogen atoms, and 50 oxygen atoms on each side. pb (co3) = pbo + co2 is a decomposition reaction where one mole of lead carbonate [pb (co 3)] decomposes into one mole of lead. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of. Lead Carbonate Balanced Equation.